By: Moise Aganze and Ruth Douglas
[BUKAVU, DRC/LONDON] Children in the Democratic Republic of Congo (DRC) are still awaiting vaccines to protect them from mpox, even as case numbers among this demographic soar, health organisations say, urging the delivery of an approved vaccine to be expedited.
Mpox is just one of numerous challenges facing the DRC, where 5.6 million people have been internally displaced as a result of conflict, mainly in the east of the country. Here, a lack of basic water, sanitation and health facilities, has also given rise to other disease outbreaks such as cholera and measles.
Soaring rates of mpox in the DRC, and its spread to neighbouring countries, prompted the WHO to declare the outbreak a public health emergency of international concern (PHEIC) in August this year – a status upheld at a WHO meeting last month.
Hopes were raised with the start of an mpox vaccination campaign in eastern DRC in October, but vaccines have been slow to arrive and children remain completely excluded, SciDev.Net has learned.
“Not only are the mpox vaccines trickling in very slowly, but the majority of suspected cases and deaths are in kids and no vaccines have been made available for kids yet,” said Roz Scourse, a policy advisor for Médecins Sans Frontières (Doctors Without Borders/MSF).
This has left many parents feeling helpless.
“I was vaccinated one week before the launch of the campaign,” said Nzigire Buhendwa, a mother-of-five from Kavumu, in the Miti-Murhesa health zone, a rural area in the northeastern part of South Kivu Province.
“But what surprised us was that we realised only adults were vaccinated, without regard for our children.”
Françoise Namegabe, whose eight-year-old son has been admitted to Miti-Murhesa hospital, told SciDev.Net: “In my family, I am the only one who has been vaccinated. I believe that if my children had received the vaccine, this would have stopped them from getting sick.”
The worries of parents are borne out in the limited disease data available.
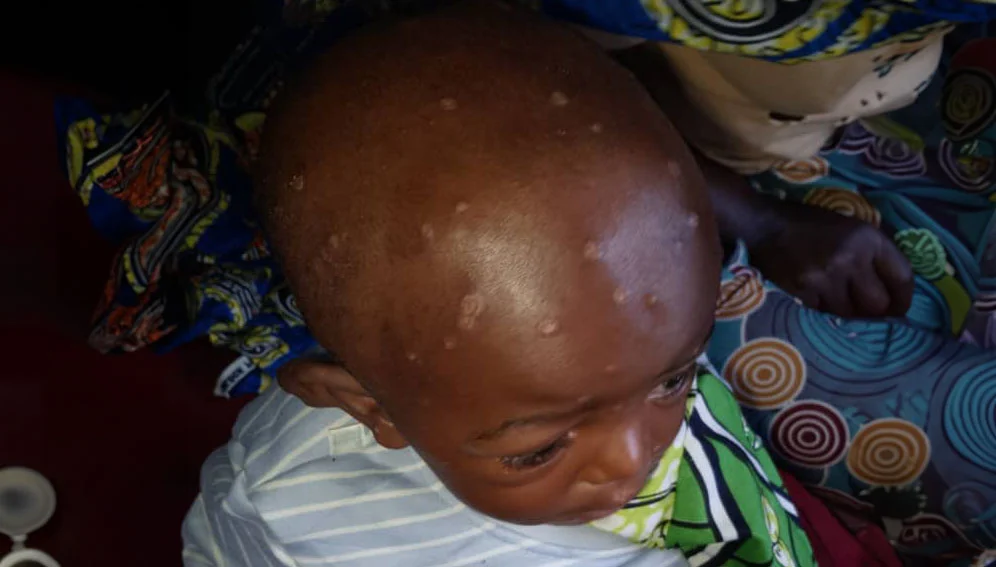
Between 14 August—the day the WHO declared the outbreak a PHEIC—and 3 November, the number of child cases of mpox in DRC more than doubled, from around 11,300 to 25,600, according to Save the Children, an NGO.
“It is children who are the most affected, especially in health zones such as Miti-Murhesa, Nyangezi, Uvira and Kamituga,” explained Claude Bazibuhe, communications officer for the Provisional Health Division of South Kivu.
“It is often adults who infect children and then the children start to infect each other while playing,” he explained.
Some babies are also being born with mpox, after being infected by the mother during pregnancy, Bazibuhe added.
Adding to the vulnerability of children to the disease are widespread malnutrition, promiscuity, the presence of other infectious diseases, limited access to health care and an overwhelmed and weakened health system.

A close-up of a patient’s hand during an mpox screening consultation in August 2024 at an MSF supported health centre on the outskirts of Goma, Democratic Republic of Congo. Photo credit: Michel Lunanga / MSF
Faced with this reality, many parents, like Buhendwa and Namegabe, are now speaking out.
“We ask the Congolese government to quickly consider this category of people in the vaccination response,” urged mother-of-five Buhendwa.
Health spokesman Bazibuhe added: “We’ve received lots of complaints from parents requesting vaccination of their children for their protection.
“We would also like to vaccinate children, but unfortunately vaccines for children are not yet available.”
Vaccine approvals
The MVA-BN vaccine, made by Bavarian Nordic in Denmark and currently being distributed in the DRC, has not been approved for use for young children, although the WHO gave the green light last month for its use in children aged over 12.
A second vaccine, the Japanese-made LC16m8 mpox vaccine, was granted Emergency Use Listing by the WHO last month (11 November) and has been recommended for use in children aged over one year of age.
“The LC16m8 vaccine has been used in Japan during previous mpox outbreaks and was shown to be safe and effective, including in people with well-controlled HIV,” a WHO spokesperson said.
Its approval follows an almost three-month review process, including discussions with producers KM Biologics, the spokesperson explained.
The decision was based on information from the manufacturer and recommendation of Japan’s regulatory body, the Pharmaceuticals and Medical Devices Agency, the WHO said.
“While the timely review and decision of these vaccines is a priority, we need to also keep at the forefront the need to do so without compromising the assessment of safety, quality and efficacy and use on target populations,” the WHO spokesperson added.
The WHO said the arrival date of the LC16m8 vaccine depended on ongoing negotiations between Japan and DRC.
Growing demand
Justin Bengehya, a health official working on the mpox response in South Kivu, said the initial order for vaccines was made when only three health zones were affected. Now 33 health zones out of the 34 in the province are affected by the disease, meaning demand is far outstripping supply.
The Miti-Murhesa health zone ranks first in terms of mortality and number of cases, with children most at risk, according to Bengehya. He says this represents a “major challenge”.
“We call on the health authorities who promised us that children’s vaccines will be available shortly to look a little more into this concern,” urged Moïse Balezi Chiberege, community facilitator for the Miti-Murhesa health zone.
Bazibuhe says the government is doing all it can to get the vaccines for children.

“We reassure the population that before long, children will also be vaccinated against mpox, because to break the chain of transmission, all vulnerable people will also need to be vaccinated,” he said.
The current vaccination campaign began in early October after DRC health authorities received the first batches of vaccines offered by the European Union (200,000 doses), the United States (50,000) and GAVI, the Vaccine Alliance (15,000).
The Congolese government decided to administer two doses of vaccine to priority groups, namely health personnel, people who have been in contact with infected patients and sex workers.
Child data lacking
Meanwhile, a key population has been neglected, says Scourse, from MSF. She says the MVA vaccine has not undergone clinical trials in children—a problem that is not unique to this vaccine, she stresses.
“We see that over and over again where kids are the last to be thought about for medical research and so the lag time between them getting access to a product that adults have is much later,” said Scourse.
“That leads to a cascade of other challenges which means that they are last to get access. And in this situation … they are most at risk of dying.”
While vaccines can be approved for emergency use despite a lack of trial data, in some circumstances, Scourse says the legal mechanisms are not in place to ensure that governments are covered for any liability, especially in poor settings.
She highlights the inequity around use of the vaccines in different countries, with the MVA mpox vaccine having been previously used to protect young children in the US and EU, but not in the countries most affected.
This piece was produced by SciDev.Net’s Sub-Saharan African French and Global desks.